| Title | A multicentre integration of a computer-led follow-up of prostate cancer is valid and safe |
|---|
| Authors | Salem, H., Caddeo, G., McFarlane, J., Patel, K., Cochrane, L., Soria, D., Henley, M. and Lund, J. |
|---|
| Abstract | Background
Prostate cancer (CaP) has a rising number of patients requiring routine follow up. In this study, we aimed to test a computer led follow up service for prostate cancer in two UK hospitals. The testing aimed to validate the computer Expert system in making clinical decisions according to the individual patient’s clinical need. The valid model should accurately identify patients with disease recurrence or treatment failure based on their blood test and clinical picture.
Methods
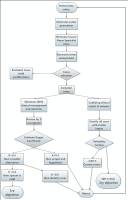
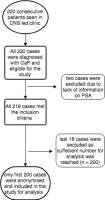
A clinical decision support system (CDSS) was developed from European (EAU) and national (NICE) guidelines along with knowledge acquired from Urologists. This model was then applied in two UK hospitals to review patients post CaP treatment. These patients’ data (n= 200) were then reviewed by two independent Urology consultants (blinded from the CDSS and other consultant’s rating) and the agreement was calculated by kappa statistics for validation. The second objective aimed to verify the system by estimating the system reliability.
Results
The two individual urology consultants identified 12 % & 15% of the patients to have potential disease progression and recommended their referral to the Urology care. The kappa coefficient for the agreement between the CDSS and the 2 consultants was 0.81 (p < 0.001) and 0.84 (p < 0.001). The agreement among both specialist was also high with k = 0.83 (p < 0.001). The system reliability was estimated on all cases and this demonstrated 100% repeatability of the decisions.
Conclusion
The computer led follow up is a valid model for providing safe follow up for prostate cancer. |
|---|
| Keywords | Clinical decision support, Expert system, Prostate cancer follow-up, Knowledge validation, Rule-based systems, System validation and verification, #PCSM, #ProstateCancer |
|---|
| Article number | BJU14157 |
|---|
| Journal | BJU international |
|---|
| Journal citation | 122 (3), pp. 418-426 |
|---|
| ISSN | 1464-4096 |
|---|
| Year | 2018 |
|---|
| Publisher | Wiley |
|---|
| Accepted author manuscript | |
|---|
| Publisher's version | |
|---|
| Digital Object Identifier (DOI) | https://doi.org/10.1111/bju.14157 |
|---|
| Publication dates |
|---|
| Published online | 06 Mar 2018 |
|---|
| Published in print | 06 Sep 2018 |
|---|
| License | CC BY-NC-ND 4.0 |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|
| Supplementary data or files | File |
|---|